Health Food
According to the Health Food Safety Assessment Method of Taiwan FDA, the safety category of the final product could be divided into four levels depending on the ingredient used:
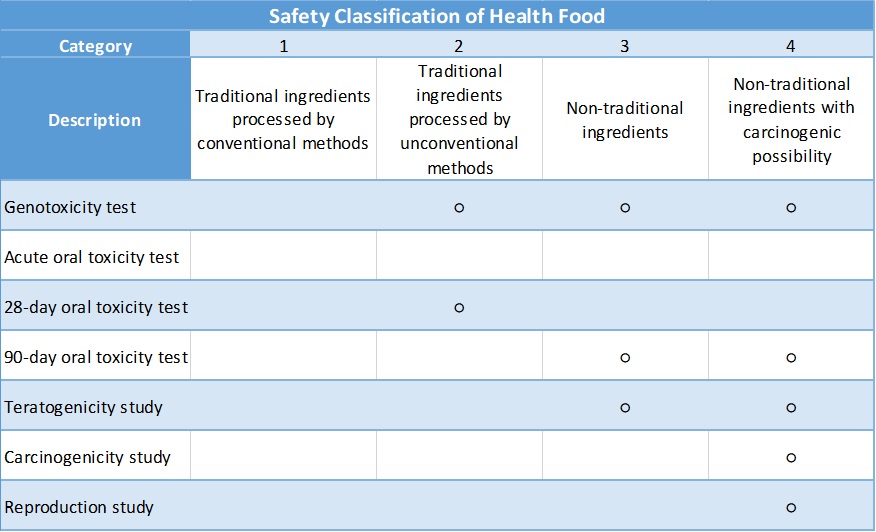
○:Required
Medgaea offers full service, including safety category evaluation and toxicity studies, to assess your products' safety, allowing our clients to address the regulatory requirements for Taiwan Health Food registration. (Contact us)
Salmonella Reverse Mutation Test
- Guideline
Health Food Safety Assessment Method
OECD 471: Bacterial Reverse Mutation Test
- Test system
Salmonella typhimurium
- Aim
To evaluate the mutagenic potential of the test substance by using bacteria in the presence or absence of rat liver enzyme metabolic system (S9 mix).
Acute Oral Toxicity Test
- Guideline
OECD 423: Acute Oral toxicity - Acute Toxic Class Method
- Test system
SD rats
- Aim
To observe the adverse effects that occurred within a short time following oral administration of the test article and evaluate the LD50 value (median lethal oral dose) of the test article.
Repeated Dose 90-Day Oral Toxicity Study in Rodents
- Guideline
Health Food Safety Assessment Method
OECD 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents
- Test system
SD rats
- Aim
To investigate the potential systemic toxicity of the test article on mammals after repeated feeding for 90 days, and to assess the No-Observed-Adverse-Effect Level (NOAEL).
Teratogenicity Study
- Guideline
Health Food Safety Assessment Method
OECD 414: Prenatal developmental toxicity study
- Test system
Pregnant female SD rats
- Aim
To assess the effects of test substances on embryonic development
Repeated Dose 28-Day Oral Toxicity Study in Rodents
- Guideline
Health Food Safety Assessment Method
OECD 407: Repeated Dose 28-day Oral Toxicity Study in Rodents
- Test system
SD rats
- Aim
To investigate the potential systemic toxicity of the test article on mammals after repeated feeding for 28 days, and to assess the No-Observed-Adverse-Effect Level (NOAEL).