- Guidance
USP 88 Biological Reactivity Tests, In Vivo
- Animals
New Zealand white rabbits
- Extraction solvent
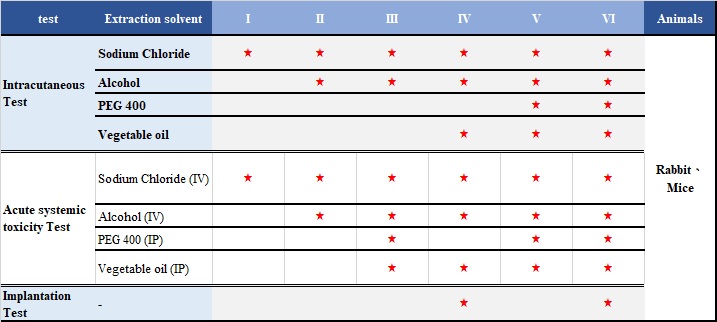
sodium chloride injection、alcohol in sodium chloride injection、polyethylene glycol 400、ottonseed oil
USP 88-Biocompatibility Tests of Raw Materials
United States Pharmacopeia, USP 88 are designed to determine the biological response of animals to raw materials.
The medical device raw materials (such as elastomerics, plastics and other polymeric materials) can be classified into Six Classes based on responses to a series of in vivo tests for which extracts, materials and routes of administration are specified. The most rigorous specification of these tests is Class VI.
There are three tests in USP 88: Intracutaneous Test、Acute systemic toxicity Test、Implantation Test。


- Guidance
USP 88 Biological Reactivity Tests, In Vivo
- Animals
Mice
- Extraction solvent
sodium chloride injection、alcohol in sodium chloride injection、polyethylene glycol 400、ottonseed oil

- Guidance
USP 88 Biological Reactivity Tests, In Vivo
- Animals
New Zealand white rabbits