- Guidelines
OECD 423
- Test system
SD rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through oral route to intake into the body.
- Lead time
8 weeks
New Chemical
Chemicals were evaluated their total amount per year as the registration level The amounts were mining through yearly estimation. The manufacture/import amount and substance will be classified as standard registration, simplified registration, and small quantity registration
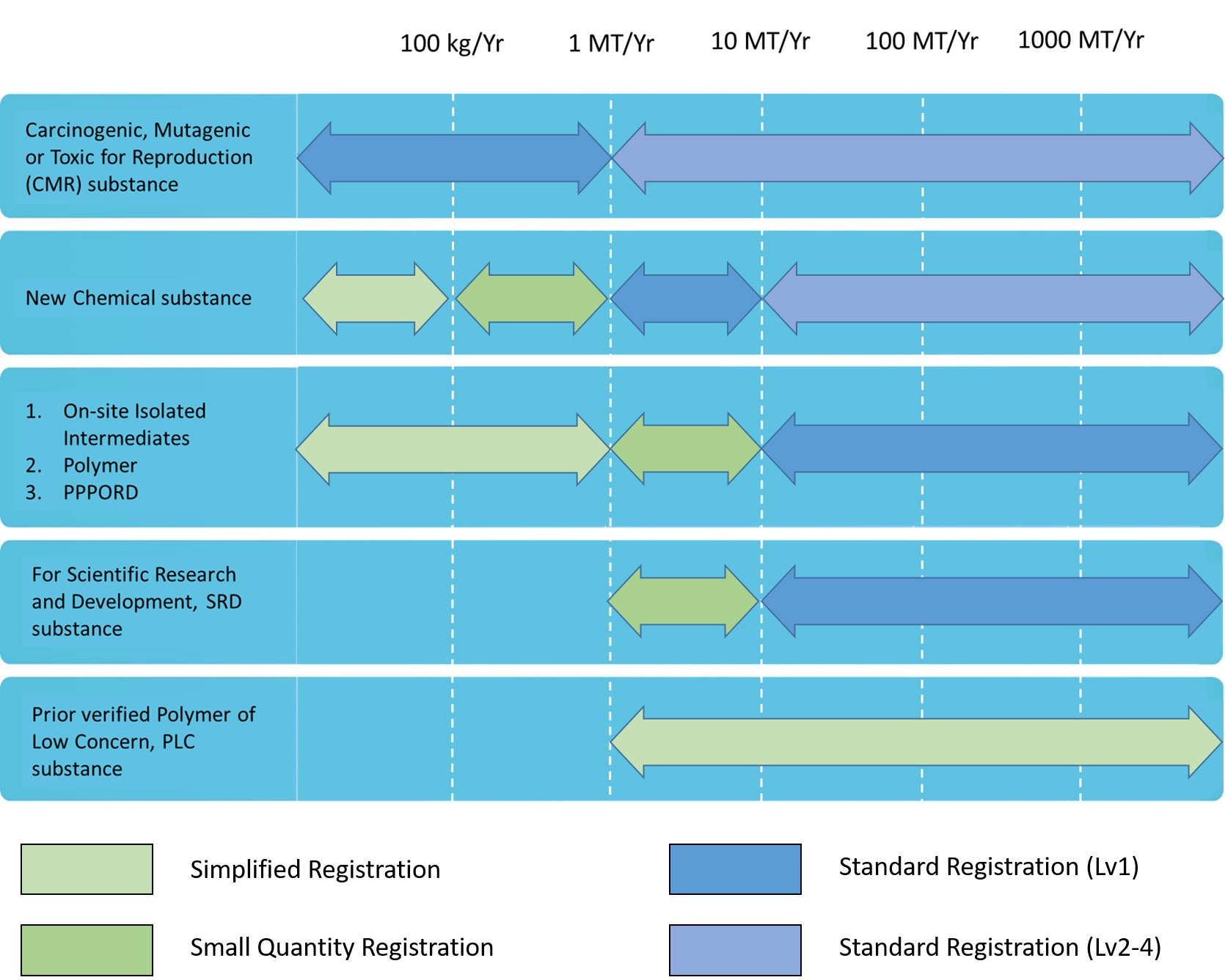
According to Guidance, after registration applications have been approved, the registrants shall proactively provide supplementary information based on the quantity thresholds when the actual manufactured or imported quantity increase leads to an increase in levels.
| Section | Items | Registration | |||||
|---|---|---|---|---|---|---|---|
| Small | Simplified | Standard (Level) | |||||
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | ||||
| 1 | General Information | ● | ● | ● | ● | ● | ● |
| 2 | Manufacture, Use and Exposure | ● | ● | ● | ● | ● | ● |
| 3 | Classification and Labelling | ● | ● | ● | ● | ● | |
| 4 | Guidance on Safe Use | ● | ● | ● | ● | ● | |
| 5 | Physical and Chemical Properties | ● | ● | ● | ● | ● | |
| 6 | Toxicological Information | ● | ● | ● | ● | ||
| 7 | Ecotoxicological Information | ● | ● | ● | ● | ||
| 8 | Hazard Assessment | ● | ● | ● | |||
| 9 | Exposure Assessment | ● | ● | ● | |||
The acquirements in “Physical and Chemical Properties”, the Section 5, through different levels of standard registration
| Items | Standard Registration | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| State of the substance | ● | ● | ● | ● |
| Melting point/freezing point | ● | ● | ● | ● |
| Boiling point | ● | ● | ● | ● |
| Density | ● | ● | ● | ● |
| Partition coefficient: n-octanol/water | ● | ● | ● | ● |
| Water solubility | ● | ● | ● | ● |
| Vapor pressure | ● | ● | ● | ● |
| Flash point | ● | ● | ● | ● |
| Flammability | ● | ● | ● | ● |
| Explosive properties | ● | ● | ● | ● |
| Oxidizing properties | ● | ● | ● | ● |
| pH | ● | ● | ● | ● |
| Auto-ignition temperature | ● | ● | ● | ● |
| Viscosity | ● | ● | ||
| Corrosive to metals | ● | ● | ||
| Items | Standard Registration | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| Acute Toxicity (Oral, Dermal, Inhalation) |
● | ● | ● | ● |
| Acute Dermal Irritation/ Corrosion | ● | ● | ● | ● |
| Acute Eye Irritation/ Corrosion | ● | ● | ● | ● |
| Skin Sensitization | ● | ● | ● | ● |
| Bacterial Reverse Mutation Test | ● | ● | ● | ● |
| in vitro Mammalian Chromosome Aberration Test | ● | ● | ● | |
| in vivo Mammalian Erythrocyte Micronucleus Test | ● | ● | ● | |
| Toxico-kinetics | ● | ● | ● | |
| Repeated Dose 28-Day Oral Toxicity Study in Rodents | ● | ● | ● | |
| Reproduction/Developmental Toxicity Screening Test | ● | ● | ● | |
| Repeated Dose 90-Day Oral Toxicity Study in Rodents | ● | ● | ||
| Prenatal Developmental Toxicity Study | ● | ● | ● | |
| Two-Generation Reproduction Toxicity Study | ● | |||
| Carcinogenicity Studies | ● | |||


- Guidelines
OECD402
- Test system
SD rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through dermal route to intake into the body
- Lead time
8 weeks

- Guidelines
OECD 403
- Test system
Wistar rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through inhalation route to intake into the body and observing the effects on lung and/or organs
- Lead time
4 months

- Guidelines
OECD 407
- Test system
SD rats
- Test Aims
The chemicals was fed orally for 28 day through three different (low, medium and high) dosage. To evaluate the change in the organs and toxicity after repeated feeding.
- Test items included
clinical symptoms, incidence, mortality, fundoscopy, body weight, feed consumption, feed utilization, hematology, serum biochemistry and electrolytes, urology, organ weight, macroscopic and pathological observations, etc. Finally, the results will present as the no observed adverse effect level, NOAEL.
- Lead time
7 to 10 months (pre-test was contained)

- Guidelines
OECD 408
- Test system
SD rats
- Test Aims
The chemicals was fed orally for 90 day to evaluate the long-term chronic toxicity. A longer feed period will better to understand the damage on specific tissues and organs. The three different (low, medium and high) dosage were used. To evaluate the change in the organs and toxicity after repeated feeding.
- Test items included
clinical symptoms, incidence, mortality, fundoscopy, body weight, feed consumption, feed utilization, hematology, serum biochemistry and electrolytes, urology, organ weight, macroscopic and pathological observations, etc. Finally, the results will present as the no observed adverse effect level, NOAEL.
- Lead time
12 to 14 months (pre-test was contained)

- Guidelines
OECD 414
- Test system
SD rats
- Test Aims
To realize whether long-term chemical use during pregnancy affects maternal, embryonic and offspring development during pregnancy.
- Lead time
12 to 14 months (pre-test was contained)

- Guidelines
OECD 202
- Test system
Daphnia magna
- Test Aims
Through observing the lethal rate, LD50, and no observable effect concentration (NOEC) to evaluate the hazard assessment on chemicals affected on Daphnia. sp. in aquatic environment.
- Lead time
3 to 6 months (pre-test was contained)

- Guidelines
OECD 201
- Test system
- Test Aims
Through observing the lethal rate, LD50, and no observable effect concentration (NOEC) to evaluate the hazard assessment on chemicals affected on freshwater algae in aquatic environment.
- Lead time
3 to 5 months (pre-test was contained)

- Guidelines
OECD 203
- Test system
zebra fish
- Test Aims
Fish are the highest consumers in vertebrates in aquatic environment. We use chemicals to co-cultivate with fish to evaluate the harm, obtain the half-effect concentration (EC50) or no observable effect concentration (NOEC) through mortality.
- Lead time
3 to 6 months (pre-test was contained)

- Guidelines
OECD 301F
- Test system
Microbiome
- Test Aims
Incubating the microbiome with chemicals to evaluate the bio-degradation effect and the inhibition effect on microbiome growth.
- Lead time
4 to 6 months