Non-traditional Food Ingredients Application in Taiwan
Introduction
According to the Guidance on Application for Non-traditional Food Ingredients, the term “non-traditional food ingredients” refers to:
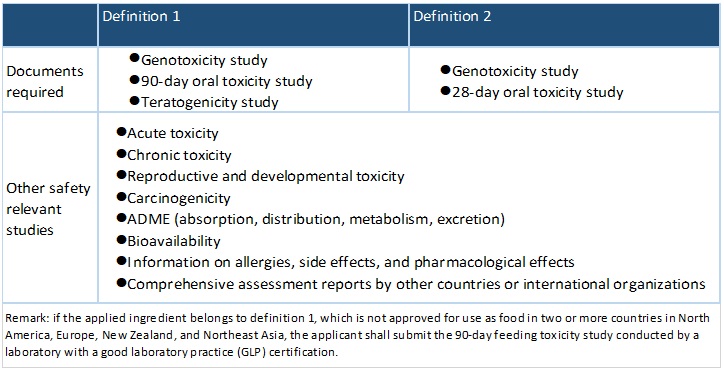
Definition 1
Ingredient that does not have a history of safe food use in Taiwan; or, does have a history of food use but not used for human consumption to a significant degree, for example, only in a specific territory or among a particular group of people
Definition 2
Traditional food materials that are produced with non-traditional breeding, planting methods or manufactured by novel processes that change the composition or properties of food (does not include genetically modified food or irradiated food).
Therefore, safety assessment is required to confirm that the novel food ingredients do not pose a health hazard, including comprehensive data collection, risk assessment, toxicology studies, etc.
Determination of Non-traditional Food Ingredients
To determine whether the ingredient is novel or not and to which definition of non-traditional food ingredients it belongs, applicants could fill out a questionnaire, prepare the relevant documents, and submit it to the TFDA for assessment. The determination could be as follows:
1. Traditional food material: no further safety assessment is required.
2. Medical ingredient: application rejected .
3. Chinese herbal medicine: need to consult the department of Chinese Medicine and Pharmacy of the Ministry of Health and Welfare for further assessment.
4. Non-traditional food ingredients: prepare the documents and information required according to the category determined.
Toxicology Information required
Other Document Required
1. Application form
2. Information of the applicant.
3. Basic information of the non-traditional food ingredient.
4. Consumption information of the non-traditional food ingredient.
5. oxicological information and other relevant information that may prove safety.
6.Labeling and instruction manual such as proposed uses, suggested for target consumers and excluded consumers, precaution and restriction of use .
7. Approval or rejection situation in other countries, in particular their relevant laws and regulations. Regulatory information in other countries.
8. Other necessary document.
Our services
The entire registration process of non-traditional food ingredients requires considerable time and continuous effort. Medgaea provides well-rounded services, from toxicity studies in compliance with GLP to comprehensive consulting and applying services. If you need assistance in novel food ingredient registration in Taiwan, welcome to contact us.
Contact Us
Feel free to contact us