Health Food Registration in Taiwan
Introduction
In Taiwan, health food is different from food supplements. Since Health Food Control Act was enacted in 1999, Health Food has become a legal term. Only the food product that has been scientifically proven to improve people’s health, and obtained the Health Food permit from TFDA, could label and advertise itself as a Health Food.
The approved one could label the reference number of the permit, the term "Health Food" and its logo, and the approved health claim on the package. However, the applicant still needs to notice that no Health Food labeling or advertisement shall extend beyond the authorized scope and shall not misrepresent, exaggerate, or refer to medical efficacy.
Steps and Tracks of Health Food Registration
There are two steps for Health Food registration:
Step 1. PreIiminary review
To review the completeness of the documents and information submitted, particulars of the applicant, package and labeling of the product, general consumption safety of ingredients of the product, etc. This step is mainly reviewed by the Center for Drug Evaluation (CDE) which is outsourced by TFDA.
Step 2. Secondary review
To review the scientific evidence of efficacy, safety, and stability reports submitted. This step is in charged by the Health Food Review Committee. After then, TFDA will notify the applicant that the application is rejected or accepted. If the case is accepted and passes the product verification conducted by TFDA, the applicant will receive the Health Food permit.
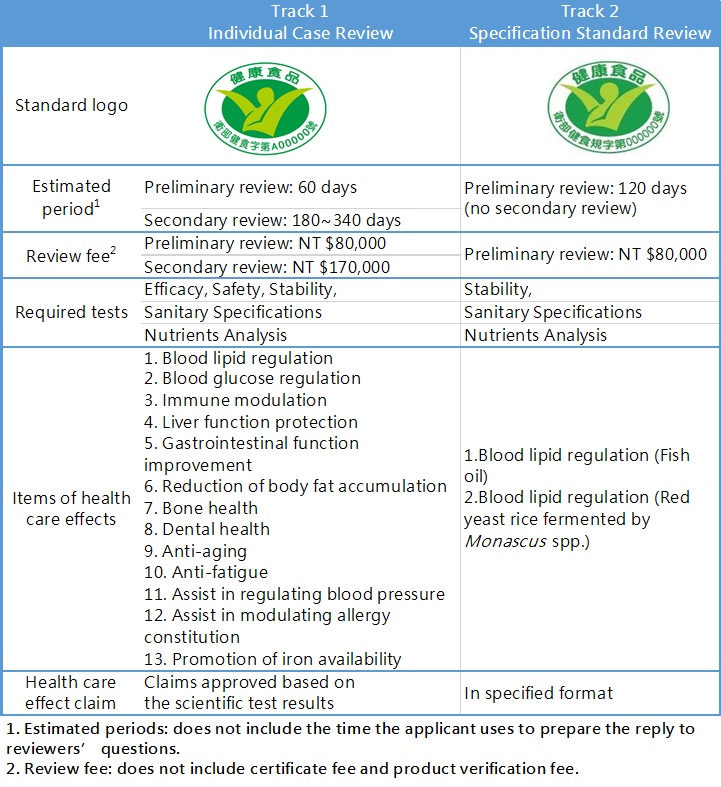
Depending on the marketing strategy and the product characteristic, there are two tracks of Health Food registration for the applicant to choose from:
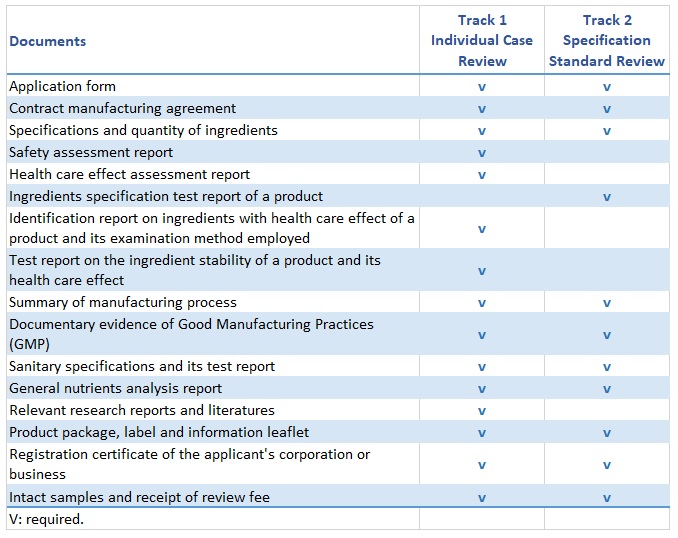
Documents Required for Health Food Registration
Maintenance of Health Food Permit
The permit shall be valid for five years and be expanded within three months before expiration. Therefore, we also provides registration services for the extension, alteration, transference and reissuance of Health Food Permits.
Our Service
Medgaea provides one-stop service of Taiwan Health Food registration, including efficacy studies, GLP toxicology assessment, stability tests, registration consulting, and filing, allowing our clients to address the up-to-date regulatory requirements from Taiwan FDA.
We provide total solutions for your products based on more than ten years of experience and our project management approach. Have a need or interest in the Taiwan Health Food application? Welcome to contact us.
Contact Us
Feel free to contact us