隨著全球工業產業發展,世界各地對於化學物質的安全性越趨重視,紛紛制定規範以追蹤化學物質再市場上的流向與安全資訊,用以保障人民的安全性以及防範對環境的影響性。
麥德凱生科是一高規格、高品質、高專業的檢驗單位,也是台灣及國外認可的檢驗公司,擁有TAF ISO17025及OECD-GLP實驗室規範認證,隨時跟進法規規範的試驗內容,無論毒理試驗或是生態毒理試驗,提供標準登錄第一級~第四級全面性試驗。除此之外,亦與其他經驗豐富且高品質的試驗單位合作,共同協助廠商於法規規範期限內順利完成。
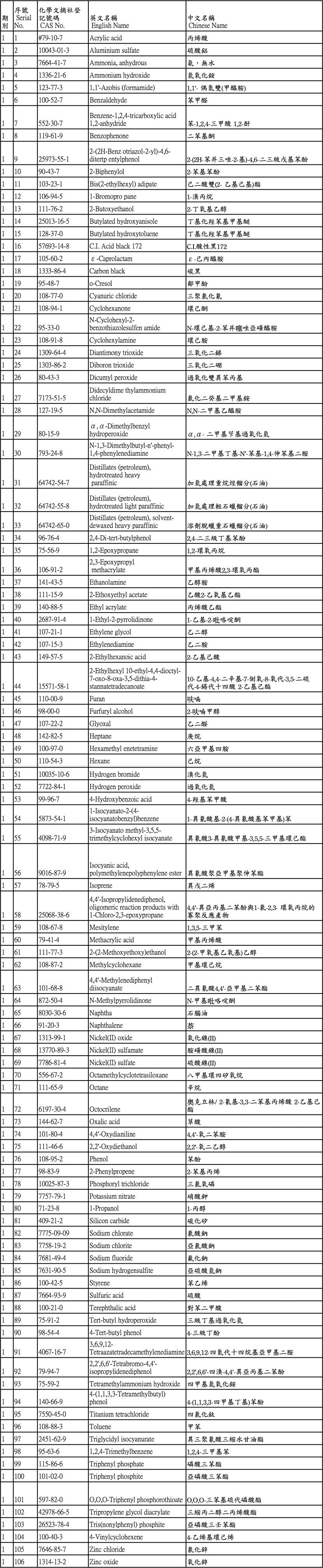
麥德凱擁有新化學物質標準登錄第三級的經驗及多家新化學物質第一級和第二級的試驗經驗,有高規格的豐富經驗,能協助客戶進行新化學物質和既有化學物質之試驗執行,既有化學物質共計106種: