Non-traditional Food Ingredients
According to Taiwan FDA, with the development of technology and the increasing international trade, more and more non-traditional food ingredients are available. In addition, the compositions and properties of many traditional foods have been changed due to non-traditional cultivation, reproduction, or new processing technology. These are all considered non-traditional food ingredients, which must undergo safety assessments to confirm that they do not constitute a health hazard.
Under the definition of non-traditional food ingredients defined by the Taiwan FDA, safety assessments requested for the application of non-traditional food ingredients are as follows:
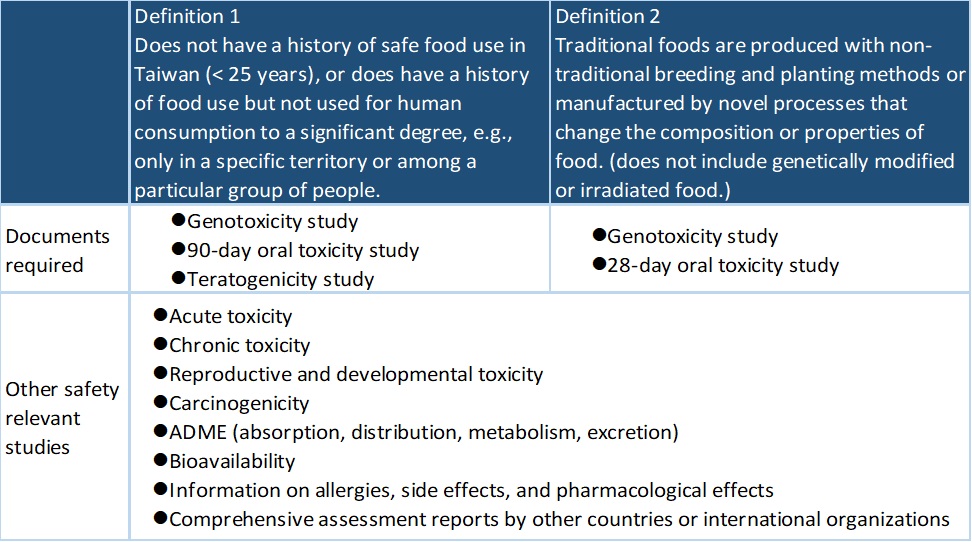
Medgaea provides GLP toxicity studies and registration consultants to assist our clients in addressing the regulatory requirements for applying non-traditional food ingredients. Welcome to contact us.
Salmonella Reverse Mutation Test
- Guideline
Health Food Safety Assessment Method
OECD 471: Bacterial Reverse Mutation Test
- Test system
Salmonella typhimurium
- Aim
To evaluate the mutagenic potential of the test substance by using bacteria in the presence or absence of rat liver enzyme metabolic system (S9 mix).
Repeated Dose 28-Day Oral Toxicity Study in Rodents
- Guideline
Health Food Safety Assessment Method
OECD 407: Repeated Dose 28-day Oral Toxicity Study in Rodents
- Test system
SD rats
- Aim
To investigate the potential systemic toxicity of the test article on mammals after repeated feeding for 28 days, and to assess the No-Observed-Adverse-Effect Level (NOAEL).
Repeated Dose 90-Day Oral Toxicity Study in Rodents
- Guideline
Health Food Safety Assessment Method
OECD 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents
- Test system
SD rats
- Aim
To investigate the potential systemic toxicity of the test article on mammals after repeated feeding for 90 days, and to assess the No-Observed-Adverse-Effect Level (NOAEL).
Acute Oral Toxicity Test
- Guideline
OECD 423: Acute Oral toxicity - Acute Toxic Class Method
- Test system
SD rats
- Aim
To observe the adverse effects that occurred within a short time following oral administration of the test article and evaluate the LD50 value (median lethal oral dose) of the test article.
Teratogenicity Study
- Guideline
Health Food Safety Assessment Method
OECD 414: Prenatal developmental toxicity study
- Test system
Pregnant female SD rats
- Aim
To assess the effects of test substances on embryonic development