- Guidance
ISO 10993-3、ISO 10993-33
- Materials
Salmonella typhimurium
- Extraction solvent
Sterile water、DMSO or Ethanol
The Biocompatibility Tests ISO 10993
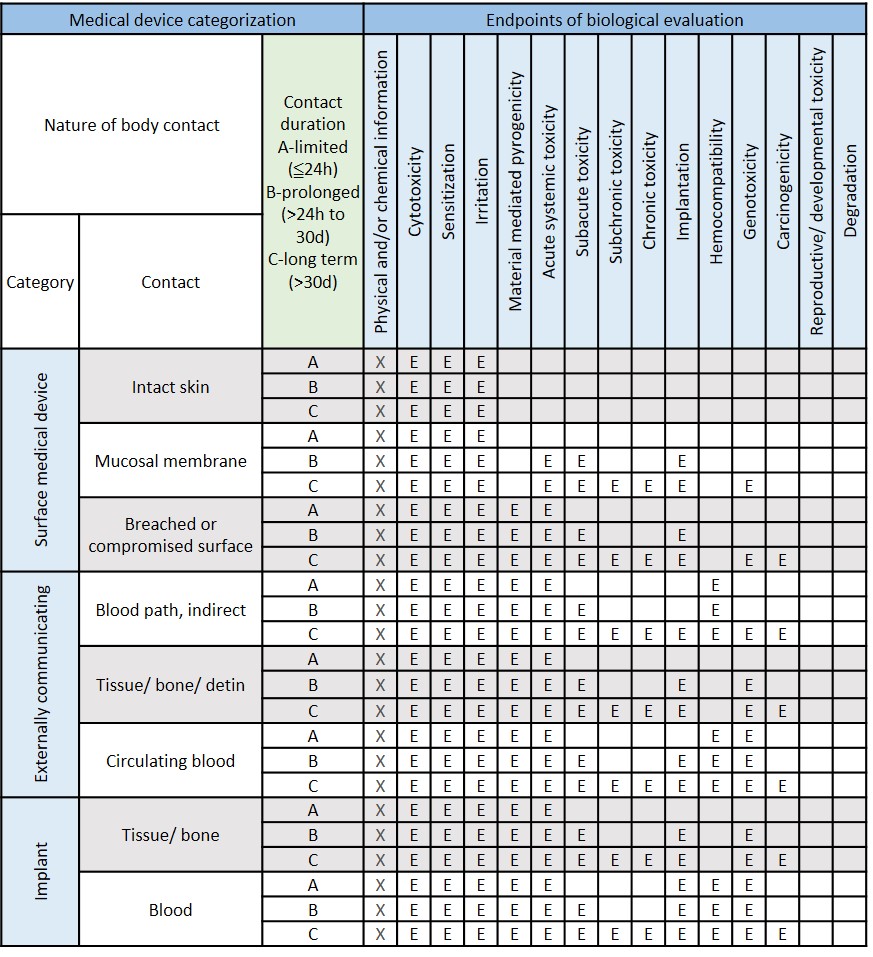
Modified from ISO 10993-1:2018 Table A.1-Endpoints to be addressed in a biological risk assessment
Salmonella Reverse Mutation Test 

In Vitro Mammalian Chromosomal Aberration test 

- Guidance
ISO 10993-3、ISO 10993-33
- Materials
CHO cell
- Extraction solvent
Culture medium without serum、Culture medium with serum
Rodent Micronucleus Test in Peripheral Blood 

- Guidance
ISO 10993-3、ISO 10993-33
- Animals
ICR mice
- Extraction solvent
Normal Saline、Cottonseed Oil
Acute Systemic Toxicity Study in (Mice, Rats, etc.) 

- Guidance
ISO 10993-11
- Animals
mice
- Extraction solvent
Normal Saline、Cottonseed Oil
Repeated exposure systemic toxicity Test (sub-acute, sub-chronic, chronic) 

- Guidance
ISO 10993-11
- Animals
Rat
- Extraction solvent
Normal Saline/ Cottonseed Oil/ or by implant directly
Implantation test 

- Guidance
ISO 10993-6
- Animals
Rat, Rabbit