New Chemical
Chemicals were evaluated their total amount per year as the registration level The amounts were mining through yearly estimation. The manufacture/import amount and substance will be classified as standard registration, simplified registration, and small quantity registration
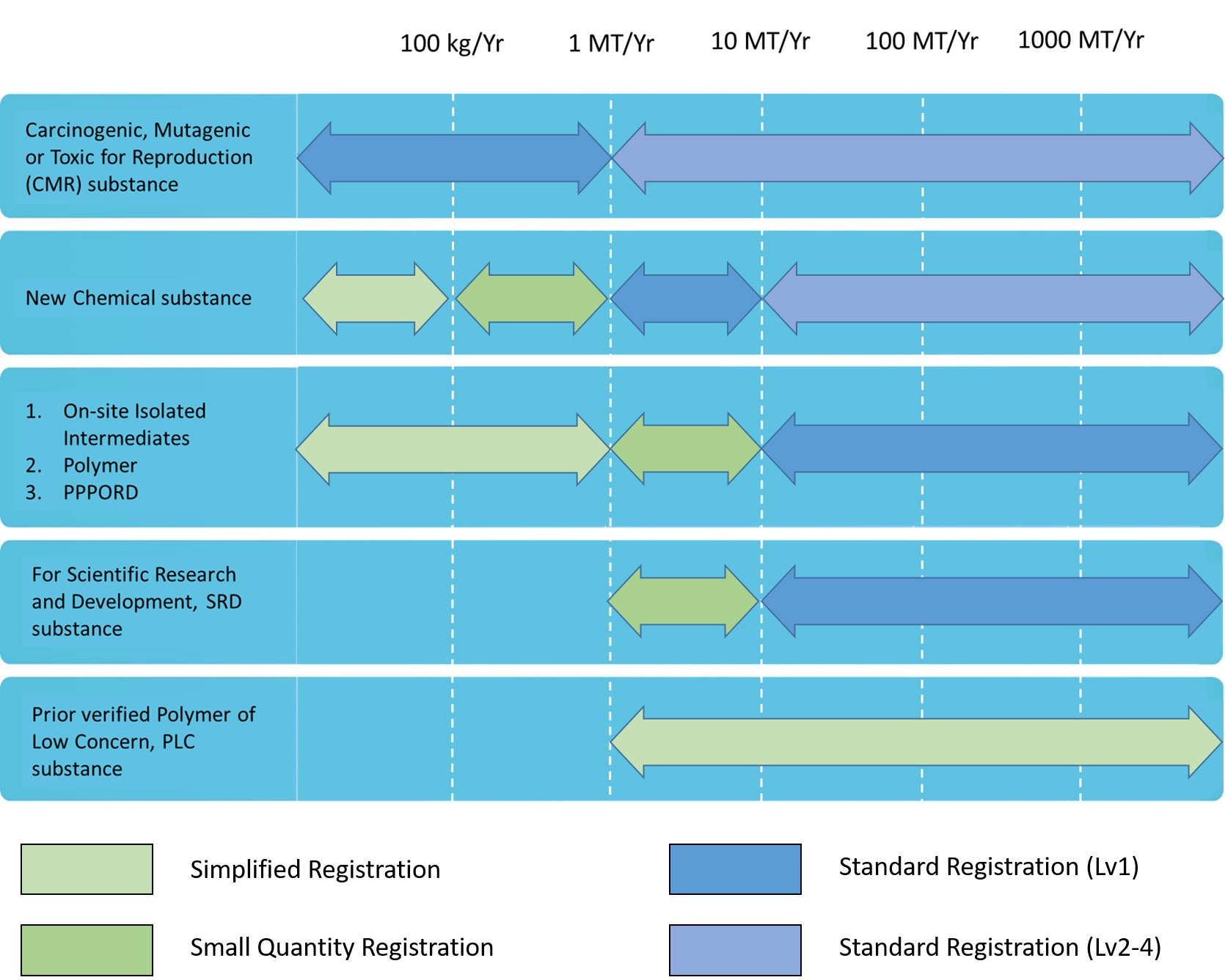
According to Guidance, after registration applications have been approved, the registrants shall proactively provide supplementary information based on the quantity thresholds when the actual manufactured or imported quantity increase leads to an increase in levels.
| Section | Items | Registration | |||||
|---|---|---|---|---|---|---|---|
| Small | Simplified | Standard (Level) | |||||
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | ||||
| 1 | General Information | ● | ● | ● | ● | ● | ● |
| 2 | Manufacture, Use and Exposure | ● | ● | ● | ● | ● | ● |
| 3 | Classification and Labelling | ● | ● | ● | ● | ● | |
| 4 | Guidance on Safe Use | ● | ● | ● | ● | ● | |
| 5 | Physical and Chemical Properties | ● | ● | ● | ● | ● | |
| 6 | Toxicological Information | ● | ● | ● | ● | ||
| 7 | Ecotoxicological Information | ● | ● | ● | ● | ||
| 8 | Hazard Assessment | ● | ● | ● | |||
| 9 | Exposure Assessment | ● | ● | ● | |||
The acquirements in “Physical and Chemical Properties”, the Section 5, through different levels of standard registration
| Items | Standard Registration | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| State of the substance | ● | ● | ● | ● |
| Melting point/freezing point | ● | ● | ● | ● |
| Boiling point | ● | ● | ● | ● |
| Density | ● | ● | ● | ● |
| Partition coefficient: n-octanol/water | ● | ● | ● | ● |
| Water solubility | ● | ● | ● | ● |
| Vapor pressure | ● | ● | ● | ● |
| Flash point | ● | ● | ● | ● |
| Flammability | ● | ● | ● | ● |
| Explosive properties | ● | ● | ● | ● |
| Oxidizing properties | ● | ● | ● | ● |
| pH | ● | ● | ● | ● |
| Auto-ignition temperature | ● | ● | ● | ● |
| Viscosity | ● | ● | ||
| Corrosive to metals | ● | ● | ||
| Items | Standard Registration | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| Acute Toxicity (Oral, Dermal, Inhalation) |
● | ● | ● | ● |
| Acute Dermal Irritation/ Corrosion | ● | ● | ● | ● |
| Acute Eye Irritation/ Corrosion | ● | ● | ● | ● |
| Skin Sensitization | ● | ● | ● | ● |
| Bacterial Reverse Mutation Test | ● | ● | ● | ● |
| in vitro Mammalian Chromosome Aberration Test | ● | ● | ● | |
| in vivo Mammalian Erythrocyte Micronucleus Test | ● | ● | ● | |
| Toxico-kinetics | ● | ● | ● | |
| Repeated Dose 28-Day Oral Toxicity Study in Rodents | ● | ● | ● | |
| Reproduction/Developmental Toxicity Screening Test | ● | ● | ● | |
| Repeated Dose 90-Day Oral Toxicity Study in Rodents | ● | ● | ||
| Prenatal Developmental Toxicity Study | ● | ● | ● | |
| Two-Generation Reproduction Toxicity Study | ● | |||
| Carcinogenicity Studies | ● | |||
Daphnia sp. Acute Immobilisation Test
- Guidelines
OECD 202
- Test system
Daphnia magna
- Test Aims
Through observing the lethal rate, LD50, and no observable effect concentration (NOEC) to evaluate the hazard assessment on chemicals affected on Daphnia. sp. in aquatic environment.
- Lead time
3 to 6 months (pre-test was contained)
Freshwater Alga and Cyanobacteria, Growth Inhibition Test
- Guidelines
OECD 201
- Test system
- Test Aims
Through observing the lethal rate, LD50, and no observable effect concentration (NOEC) to evaluate the hazard assessment on chemicals affected on freshwater algae in aquatic environment.
- Lead time
3 to 5 months (pre-test was contained)
Fish, Acute Toxicity Test
- Guidelines
OECD 203
- Test system
zebra fish
- Test Aims
Fish are the highest consumers in vertebrates in aquatic environment. We use chemicals to co-cultivate with fish to evaluate the harm, obtain the half-effect concentration (EC50) or no observable effect concentration (NOEC) through mortality.
- Lead time
3 to 6 months (pre-test was contained)
Ready Biodegradability: Manometric Respirometry Test
- Guidelines
OECD 301F
- Test system
Microbiome
- Test Aims
Incubating the microbiome with chemicals to evaluate the bio-degradation effect and the inhibition effect on microbiome growth.
- Lead time
4 to 6 months
Acute Oral toxicity
- Guidelines
OECD 423
- Test system
SD rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through oral route to intake into the body.
- Lead time
8 weeks