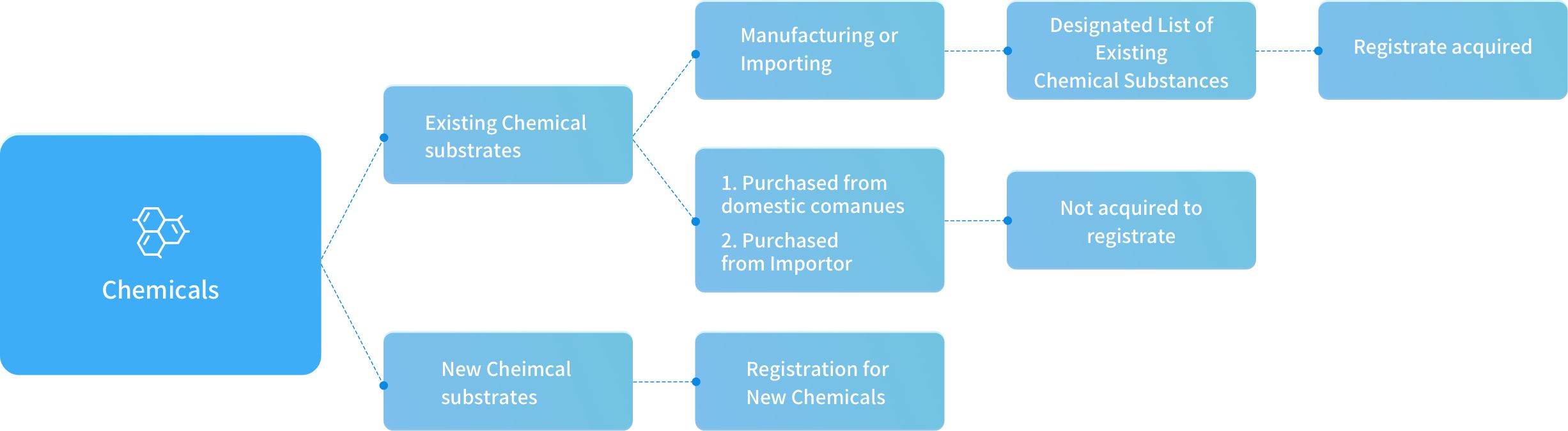
Existing Chemical
The evaluating standard for standard registration
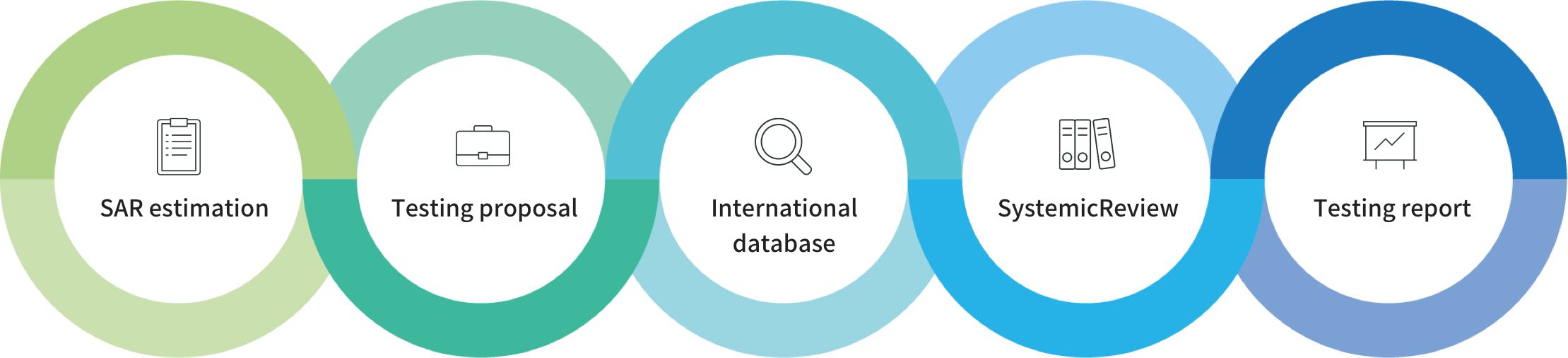
The acceptance for the data
File download
Acute Oral toxicity
- Guidelines
OECD 423
- Test system
SD rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through oral route to intake into the body.
- Lead time
8 weeks
Acute Dermal toxicity
- Guidelines
OECD402
- Test system
SD rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through dermal route to intake into the body
- Lead time
8 weeks
Acute Inhalation toxicity
- Guidelines
OECD 403
- Test system
Wistar rats
- Test Aims
Evaluating the lethal dosage and LD50 of the chemicals through inhalation route to intake into the body and observing the effects on lung and/or organs
- Lead time
4 months
28 day repeated dosage by oral
- Guidelines
OECD 407
- Test system
SD rats
- Test Aims
The chemicals was fed orally for 28 day through three different (low, medium and high) dosage. To evaluate the change in the organs and toxicity after repeated feeding.
- Test items included
clinical symptoms, incidence, mortality, fundoscopy, body weight, feed consumption, feed utilization, hematology, serum biochemistry and electrolytes, urology, organ weight, macroscopic and pathological observations, etc. Finally, the results will present as the no observed adverse effect level, NOAEL.
- Lead time
7 to 10 months (pre-test was contained)
90 day repeated dosage by oral
- Guidelines
OECD 408
- Test system
SD rats
- Test Aims
The chemicals was fed orally for 90 day to evaluate the long-term chronic toxicity. A longer feed period will better to understand the damage on specific tissues and organs. The three different (low, medium and high) dosage were used. To evaluate the change in the organs and toxicity after repeated feeding.
- Test items included
clinical symptoms, incidence, mortality, fundoscopy, body weight, feed consumption, feed utilization, hematology, serum biochemistry and electrolytes, urology, organ weight, macroscopic and pathological observations, etc. Finally, the results will present as the no observed adverse effect level, NOAEL.
- Lead time
12 to 14 months (pre-test was contained)