目前主管機關因為疫情的關係將所有級距延後至2023年底需登錄完成,真正的時間需等主管機關修法完成後公告。下表為尚未修法前的登錄期限。
|
首次取得登錄碼 日期 |
2018/12/31 以前 |
2019/01/01 以後 |
|---|---|---|
|
1~100 噸 |
2021/12/31 |
取得登錄碼以後3年 |
|
1000 噸以上 |
2020/12/31 |
取得登錄碼以後3年 |
|
About |
Medgaea Life Sciences is a third party laboratory that is certificated for OECD GLP, TFDA GLP, ISO 17025 and ISO 9001. Maily based on preclinical CRO, we provide total-soltion services to assist biotechnology industries successfully obtain the product certification and exploit internation market. |
|
CRO
|
With devoting to our core value 『Honesty•Accuracy•Professionalism•Responsibility』,
We have successfully assisted our customers to successfully obtain the product certification in FDA, CE, TFDA and NMPA.
|
999 Valued Customer |
2884 Sample tested |
2448 Saftey Evaluation tests |
44 Efficacy Evaluation tests |
392 Other Customerized tests |
Statistics by 2017-2020
Report Application Experiences
Medical Devices
Medgaea Life Sciences have assisted our customers registration worldwide (FDA, CE, TFDA, NMPA) successfully in the past decade including: Medical device raw materials (plastics, rubber, etc.), wound dressings, contact lenses, urinary catheters, intravaginal and intra-intestinal devices, skin staples, dental filling materials, intravascular catheters, nebulizers, bone cements and so on.
Chemical substance
We can provide the toxicological test which the standard registration of new chemical acquired to submit! Our test report was accepted by the authority such as EPA, CE, etc.
Agrochemicals
The test report was accepted from TACTRI. We already help several clients listing their materials such as PENOXSULAM TC, tolfenpyrad TC, QUINCLORAC TC, transfluthrin TC, Trifloxystrobin TC.
Environmental agents
Our report was accepted by EPA, the authority. We already help our clients listing their products, such as Picaridin (several concentration) and others repellents!
Food
Our reports are approved by the Taiwan Food and Drug Administration of the Ministry of Health and Welfare. The forms of test samples include capsules, tablets, powders, drinks, etc. And the types include plant extracts, microbial fermentation products, animal-derived food raw materials, mushroom-derived food raw materials, Antrodia cinnamomea, and many other categories.
Safety Evaluation Tests (In vitro/ In vivo)
We provide safety assessment tests in various industrial fields: medical devices, chemicals, pesticides and environmental agents, health food/non-traditional food raw materials, pharmas, etc. An average of 800 toxicology tests are performed each year. Our reports have been accepted by TFDA, FDA, B28CE, and NMPA.
Efficacy assessment
We’ve established various efficacy test models according to the evaluation methods of health care effects of Health Food announced by the Taiwan FDA.
目前主管機關因為疫情的關係將所有級距延後至2023年底需登錄完成,真正的時間需等主管機關修法完成後公告。下表為尚未修法前的登錄期限。
|
首次取得登錄碼 日期 |
2018/12/31 以前 |
2019/01/01 以後 |
|---|---|---|
|
1~100 噸 |
2021/12/31 |
取得登錄碼以後3年 |
|
1000 噸以上 |
2020/12/31 |
取得登錄碼以後3年 |
在2018年前首次取得登錄碼時,以當年的噸數往前推3年,3年中最高噸數。
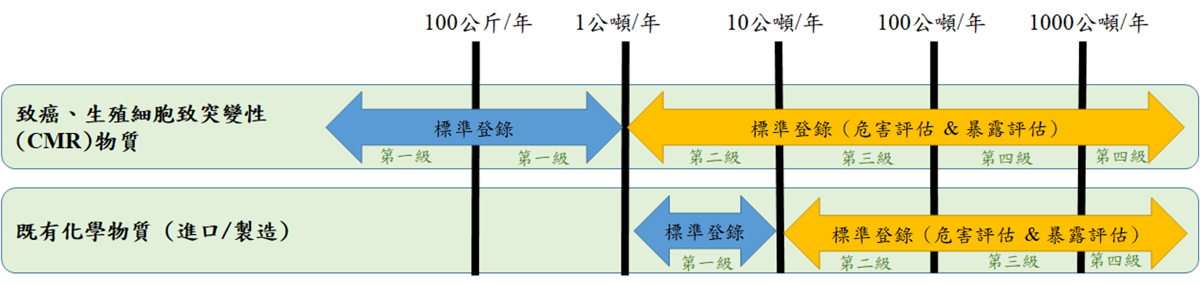
每個級距需繳交的資料不同,第一級是無需繳交危害及暴露評估,第二級到第四級的廠商,則可先提供1~7項的數據資料,在補繳危害及暴露評估資訊。
|
登錄資訊 |
第一級 |
第一級 |
第一級 |
第一級 |
|---|---|---|---|---|
| 1. 登錄人與物質辨識資訊 | V | V | V |
V |
| 2. 製造、用途與暴露資訊 | V |
V |
V |
V |
| 3. 危害分類與標示 |
V |
V |
V |
V |
| 4. 安全使用資訊 |
V |
V |
V |
V |
| 5. 物理化學特性資訊 |
V |
V |
V |
V |
| 6. 毒理資訊 |
V |
V |
V |
V |
| 7. 生態毒理資訊 |
V |
V |
V |
V |
| 8. 危害評估資訊 |
V |
V |
V |
|
| 9. 暴露評估資訊 |
V |
V |
V |
既有化學物質第一階段登錄並無效期,故不用重複登錄或展延;新化學物質則有核准登錄期限,應依登錄辦法第13條,自有效期間屆滿6個月前起算3個月之期間內提出展延申請。 而「申報」與「登錄」之作業並不相同,經核准登錄之新化學物質及既有化學物質,應依登錄辦法第24條,於核准登錄後每年4月1日至9月30日,申報前一年製造及輸入之數量資訊。
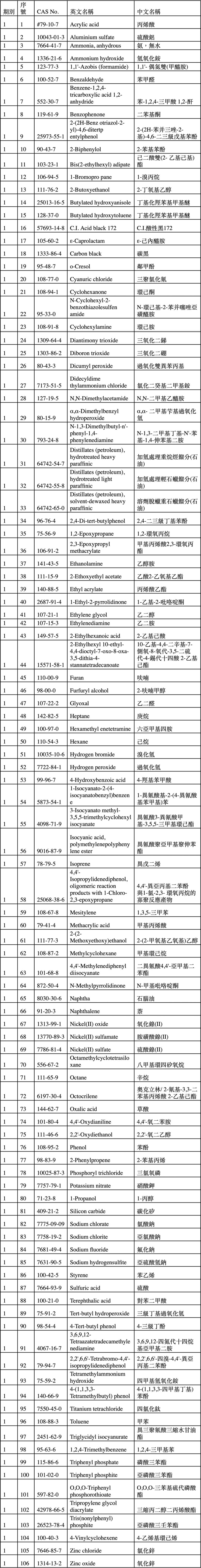
目前已公告106項化學物質為既有化學物質,如附表。
To provide more integrity service and support our clients for the international development, we got several certification since 2008. Until now, we still followed and maintained the certificates.
| Certification | Initial Year |
|---|---|
|
ISO 17025 |
2009 |
|
OECD GLP |
2011 |
|
TFDA GLP |
2009 |
|
ISO 9001:2000 |
2008 |
 |
 |
 |
 |