Uncategorised Article Count: 1
首頁 Article Count: 0
首頁slider Article Count: 2
首頁slider-m Article Count: 1
關於我們 Article Count: 2
臨床前試驗委託 Article Count: 8
測試與服務 Article Count: 0
Pharmaceutical development Article Count: 0
As the agent of Japan’s largest and oldest preclinical CRO-SNBL(Shin Nippon Biomedical Laboratories,) in Taiwan, MedGaea assist new drugs company to complete non-clinical trials in medium and large animals as well as primates in the research and development stage. We look forward to promoting the preclinical development of new drugs industries.
Preclinical tests Article Count: 17
SNBL is the first CRO in Japan and have since steadily expanded and innovated to become the world's largest non-human primate drug safety research facility. They strive to be the leader in animal welfare in Asia, providing quality scientific data while continuously focusing on the 3Rs.
Research and Development support Article Count: 4
Strong Support Systems and Qualifications
Being Japan's largest preclinical facility allows us the flexibility to accommodate a wide variety of products and study types.
Biopharmaceuticals and Cell and Therapy Products Article Count: 5
Experience in Evaluation of Biopharmaceuticals and Cell and Gene Therapy Products
- Over 2000 studies on biopharmaceuticals in the past 5 years
- Over 120 studies on cell and gene thaerapy products
New drug development and application Article Count: 4
One-Stop Service, Medical Writing, and Consulting for Drug Development
Nonclinical pharmaceutical affairs experts, study directors, and project managers who are experienced Japan and overseas countries offer a one-stop service as scientific monitors.
Staff experienced with the preparation of investigator's brochures(IB) and CTD or experienced study direcrors offer medical writing as scientific writers.
Sciencetific monitor or writers are able to consult on various matters according to your needs.
Cell therapy and Medicine Article Count: 0
MedGaea provides testing services for drugs and cell therapy products during the research and development stage.
We promised all the toxicological test followed the GLP guidance. Also, our lab have passed the Taiwan FDA inspection, we can provide batch examine for medicine batch release examine, including pyrogen, acute systemic toxicity and other customized tests. Taking care on the safety for the pharmaceutical factor and customers!
For medicine safety guidance, we offered an array of services following OECD guidelines, Japanese Pharmacopoeia, US Pharmacopoeia, and the regulatory requirements from the Taiwan FDA.
For more information, please contact us!
Batch examines Article Count: 3
Our lab have passed the Taiwan FDA inspection, we can provide batch examine for medicine batch release examine, including pyrogen, acute systemic toxicity and other customized tests. Taking care on the safety for the pharmaceutical factor and customers!
Toxicity tests Article Count: 8
Other safety tests Article Count: 1
Other Customized Test Article Count: 1
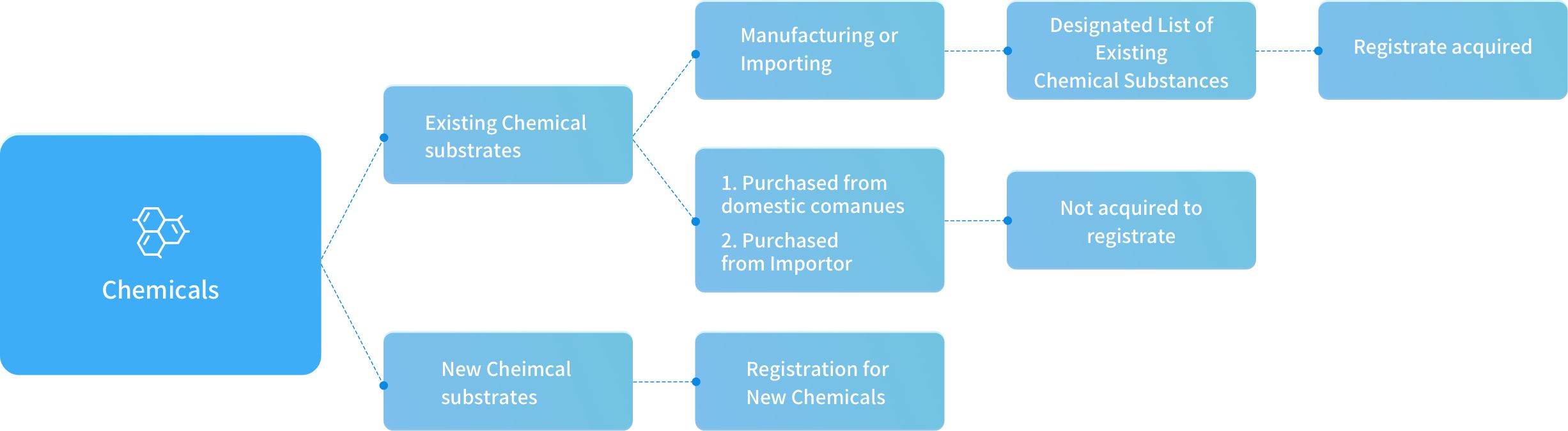
Chemicals Article Count: 0
Existing Chemical Article Count: 10
The evaluating standard for standard registration
The acceptance for the data
File download
New Chemical Article Count: 10
Chemicals were evaluated their total amount per year as the registration level The amounts were mining through yearly estimation. The manufacture/import amount and substance will be classified as standard registration, simplified registration, and small quantity registration
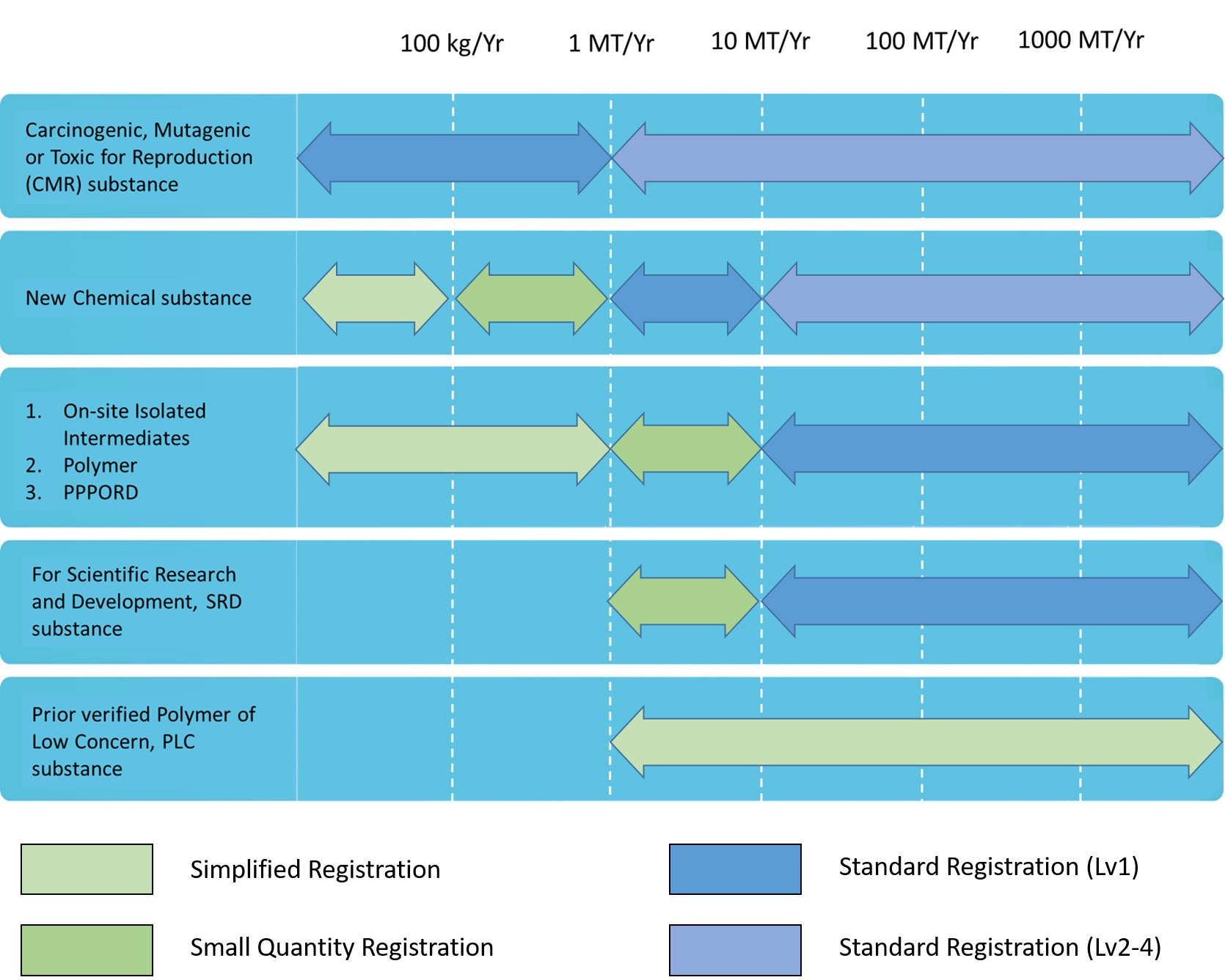
According to Guidance, after registration applications have been approved, the registrants shall proactively provide supplementary information based on the quantity thresholds when the actual manufactured or imported quantity increase leads to an increase in levels.
| Section | Items | Registration | |||||
|---|---|---|---|---|---|---|---|
| Small | Simplified | Standard (Level) | |||||
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | ||||
| 1 | General Information | ● | ● | ● | ● | ● | ● |
| 2 | Manufacture, Use and Exposure | ● | ● | ● | ● | ● | ● |
| 3 | Classification and Labelling | ● | ● | ● | ● | ● | |
| 4 | Guidance on Safe Use | ● | ● | ● | ● | ● | |
| 5 | Physical and Chemical Properties | ● | ● | ● | ● | ● | |
| 6 | Toxicological Information | ● | ● | ● | ● | ||
| 7 | Ecotoxicological Information | ● | ● | ● | ● | ||
| 8 | Hazard Assessment | ● | ● | ● | |||
| 9 | Exposure Assessment | ● | ● | ● | |||
The acquirements in “Physical and Chemical Properties”, the Section 5, through different levels of standard registration
| Items | Standard Registration | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| State of the substance | ● | ● | ● | ● |
| Melting point/freezing point | ● | ● | ● | ● |
| Boiling point | ● | ● | ● | ● |
| Density | ● | ● | ● | ● |
| Partition coefficient: n-octanol/water | ● | ● | ● | ● |
| Water solubility | ● | ● | ● | ● |
| Vapor pressure | ● | ● | ● | ● |
| Flash point | ● | ● | ● | ● |
| Flammability | ● | ● | ● | ● |
| Explosive properties | ● | ● | ● | ● |
| Oxidizing properties | ● | ● | ● | ● |
| pH | ● | ● | ● | ● |
| Auto-ignition temperature | ● | ● | ● | ● |
| Viscosity | ● | ● | ||
| Corrosive to metals | ● | ● | ||
| Items | Standard Registration | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| Acute Toxicity (Oral, Dermal, Inhalation) |
● | ● | ● | ● |
| Acute Dermal Irritation/ Corrosion | ● | ● | ● | ● |
| Acute Eye Irritation/ Corrosion | ● | ● | ● | ● |
| Skin Sensitization | ● | ● | ● | ● |
| Bacterial Reverse Mutation Test | ● | ● | ● | ● |
| in vitro Mammalian Chromosome Aberration Test | ● | ● | ● | |
| in vivo Mammalian Erythrocyte Micronucleus Test | ● | ● | ● | |
| Toxico-kinetics | ● | ● | ● | |
| Repeated Dose 28-Day Oral Toxicity Study in Rodents | ● | ● | ● | |
| Reproduction/Developmental Toxicity Screening Test | ● | ● | ● | |
| Repeated Dose 90-Day Oral Toxicity Study in Rodents | ● | ● | ||
| Prenatal Developmental Toxicity Study | ● | ● | ● | |
| Two-Generation Reproduction Toxicity Study | ● | |||
| Carcinogenicity Studies | ● | |||
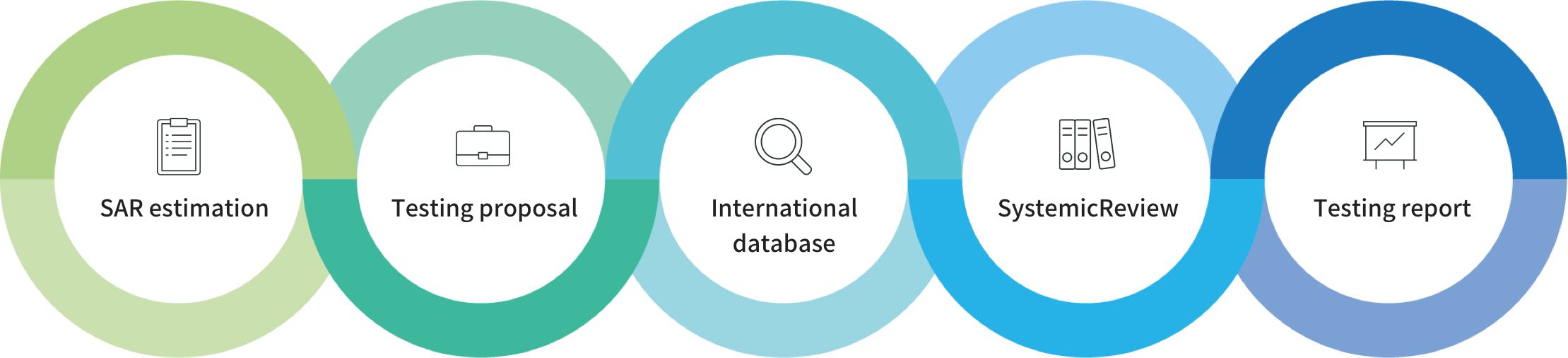
Registration Service Article Count: 3
Agrochemical and Enviromental Agents Article Count: 0
To concern our environments more, domestic suppliers devoting to research and development environmental agents (repellent) and pesticide (plant protectant) on the way to achieve the goal: low-toxicity, high-capacity, increasing efficiency, high degradable, and reducing-usage!
According to the “Regulations for the Physicochemical and Toxicological Test of Agro-pesticides”, Council of Agriculture, Executive Yuan, required the toxicology information for the safety of agrochemicals to customers, peasantry, and environments.
The suppliers were requested for their products for pre-auditing their toxicologically information. The toxicologically evaluation test must be executed under the GLP-grade laboratory.
Medgaea (MDG) laboratory achieved the GLP-grade certification and followed OECD guideline!
Our teams recruited multiple PhD-level study directors and veterinarians who had more than 10-year experienced in pre-CRO industry. Persisted the professional attitude and experiments skills, we provided complete and trustful scientific report. Finally, we assist the suppliers shorten the duration to launch!
Genotoxicity Article Count: 3
Eco-toxicological test Article Count: 3
Short-term toxicity Article Count: 6
Repeated dosage test Article Count: 1
Health/Functional Food Article Count: 0
From crop, animal, and aquatic products to functional food ingredients, food supplements, and health foods, Medgaea Life Science Ltd assists many companies in product research and development and brings value-added effects. We provide scientific evidence support to your products through safety, stability, and efficacy tests. In cooperation with Medgaea Japan, we can help to promote your products to the international health food market.
Efficacy Test Article Count: 11
As per Taiwan FDA requests, health foods that claim functions as below should obtain approval of Health Food Registration before importing, manufacturing, or launching:
- Blood lipid regulation
- Blood glucose regulation
- Immune modulation
- Liver function protection
- Gastrointestinal function improvement
- Reduction of body fat accumulation
- Bone health
- Dental health
- Anti-aging
- Anti-fatigue
- Assist in regulating blood pressure
- Assist in modulating allergy constitution
- Promotion of iron availability
Medgaea provides efficacy evaluation tests for Health Foods registration. According to the function design of the food product, we also deliver pilot studies for formula screening and dose range finding for our clients to reduce the cost and risk of new product development. Welcome to contact us.
Safety Assessment Article Count: 0
Medgaea is certificated by Taiwan Accreditation Foundation (TAF), the national GLP Compliance Monitoring Authority in Taiwan, for our OECD and Taiwan FDA Good Laboratory Practices (GLP) compliances. We offer full service to assess the safety of your products according to the Health Food Safety Assessment Method of Taiwan FDA or the Guidelines for the Testing of Chemicals of OECD.
Health Food Article Count: 7
According to the Health Food Safety Assessment Method of Taiwan FDA, the safety category of the final product could be divided into four levels depending on the ingredient used:
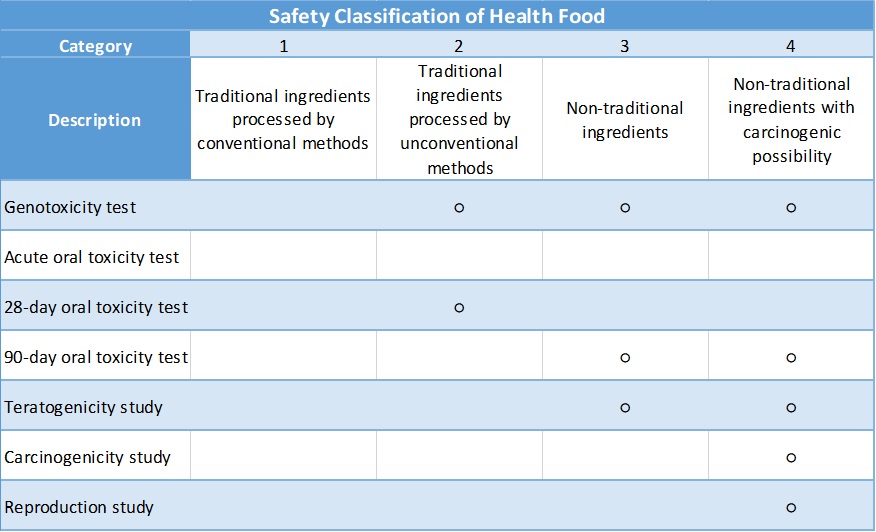
○:Required
Medgaea offers full service, including safety category evaluation and toxicity studies, to assess your products' safety, allowing our clients to address the regulatory requirements for Taiwan Health Food registration. (Contact us)
Non-traditional Food Ingredients Article Count: 7
According to Taiwan FDA, with the development of technology and the increasing international trade, more and more non-traditional food ingredients are available. In addition, the compositions and properties of many traditional foods have been changed due to non-traditional cultivation, reproduction, or new processing technology. These are all considered non-traditional food ingredients, which must undergo safety assessments to confirm that they do not constitute a health hazard.
Under the definition of non-traditional food ingredients defined by the Taiwan FDA, safety assessments requested for the application of non-traditional food ingredients are as follows:
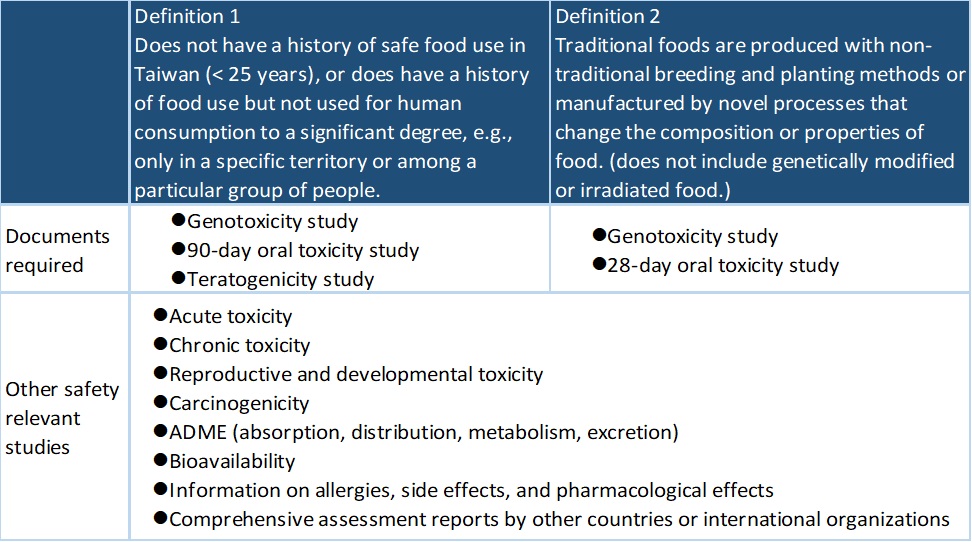
Medgaea provides GLP toxicity studies and registration consultants to assist our clients in addressing the regulatory requirements for applying non-traditional food ingredients. Welcome to contact us.
Stability Test Article Count: 5
Food's "shelf life" will be affected by factors such as the ingredients used, the manufacturing process, the storage, transportation, and sales environment, etc.; storage experiments should be designed and determined according to individual circumstances.
For Taiwan Health Food registration, stability tests of the final product are required to study the influence of environmental factors on the declared health care effect-related components and the quality of the product. The stability test results support or estimate the product's shelf-life (expiry date) and ensure the safety and efficacy it claimed within the expiration date.
Please contact us if you need stability tests for the Health Food registration.
Registration Article Count: 0
Medgaea provides consultation and filing services for food-related registrations in Taiwan following the up-to-date guidelines announced by TFDA. We have successfully assisted many companies in obtaining different kinds of permits.
If you need assistance with food registration in Taiwan, please contact us.
Health Food Registration in Taiwan Article Count: 1
Introduction
In Taiwan, health food is different from food supplements. Since Health Food Control Act was enacted in 1999, Health Food has become a legal term. Only the food product that has been scientifically proven to improve people’s health, and obtained the Health Food permit from TFDA, could label and advertise itself as a Health Food.
The approved one could label the reference number of the permit, the term "Health Food" and its logo, and the approved health claim on the package. However, the applicant still needs to notice that no Health Food labeling or advertisement shall extend beyond the authorized scope and shall not misrepresent, exaggerate, or refer to medical efficacy.
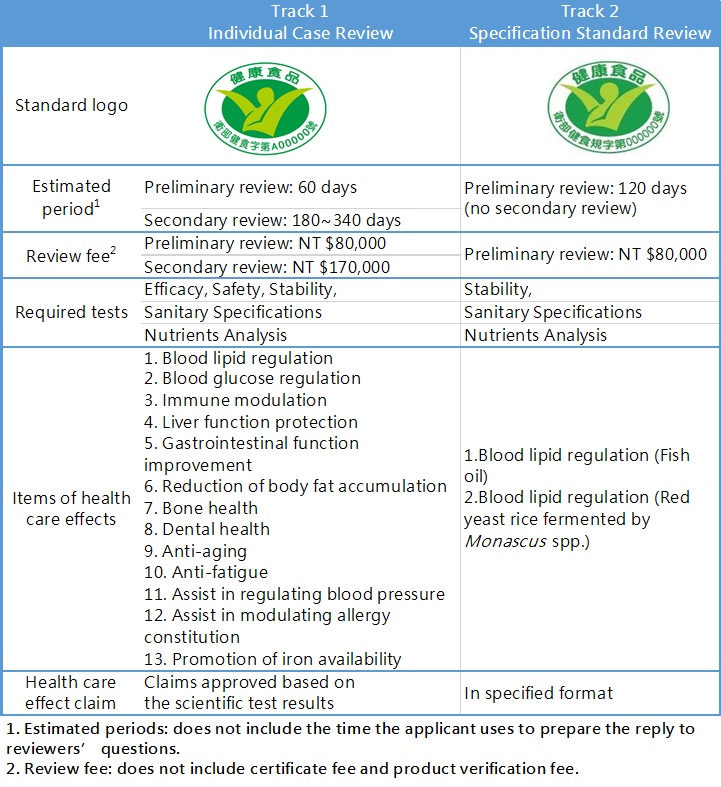
Steps and Tracks of Health Food Registration
There are two steps for Health Food registration:
Step 1. PreIiminary review
To review the completeness of the documents and information submitted, particulars of the applicant, package and labeling of the product, general consumption safety of ingredients of the product, etc. This step is mainly reviewed by the Center for Drug Evaluation (CDE) which is outsourced by TFDA.
Step 2. Secondary review
To review the scientific evidence of efficacy, safety, and stability reports submitted. This step is in charged by the Health Food Review Committee. After then, TFDA will notify the applicant that the application is rejected or accepted. If the case is accepted and passes the product verification conducted by TFDA, the applicant will receive the Health Food permit.
Depending on the marketing strategy and the product characteristic, there are two tracks of Health Food registration for the applicant to choose from:
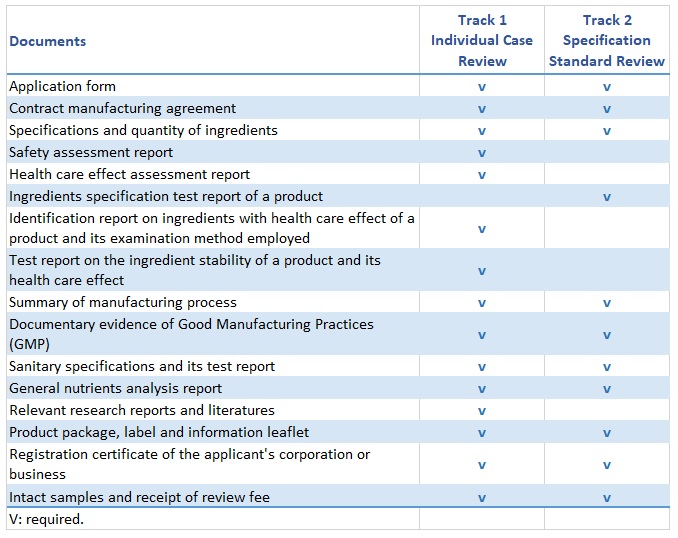
Documents Required for Health Food Registration
Maintenance of Health Food Permit
The permit shall be valid for five years and be expanded within three months before expiration. Therefore, we also provides registration services for the extension, alteration, transference and reissuance of Health Food Permits.
Our Service
Medgaea provides one-stop service of Taiwan Health Food registration, including efficacy studies, GLP toxicology assessment, stability tests, registration consulting, and filing, allowing our clients to address the up-to-date regulatory requirements from Taiwan FDA.
We provide total solutions for your products based on more than ten years of experience and our project management approach. Have a need or interest in the Taiwan Health Food application? Welcome to contact us.
Registration of Imported Food in Tablet or Capsule Form in Taiwan Article Count: 1
Introduction
As per Taiwan Ministry of Health and Welfare requests, foreign food products in tablet or capsule form should obtained approval before import. The documents and materials required for the registration includes:
1. Application form
2. Product ingredient and content table issued by the original manufacturer
3. Official certificate evidencing the legitimacy of the original manufacturer issued by the competent authority
4. Applicant’s company or business registration certificate
5. An intact sample of the product
6. Food details list
7. Affidavit
8. Registration information list
9. Other related information and Registration fee
Medgaea has a wealth of experience assisting applicants in food formula checking, Chinese labeling, and registration application. If you need further assistant in registration of imported food in tablet or capsule form, welcome to contact us.
Non-traditional Food Ingredients Application in Taiwan Article Count: 1
Introduction
According to the Guidance on Application for Non-traditional Food Ingredients, the term “non-traditional food ingredients” refers to:
Definition 1
Ingredient that does not have a history of safe food use in Taiwan; or, does have a history of food use but not used for human consumption to a significant degree, for example, only in a specific territory or among a particular group of people
Definition 2
Traditional food materials that are produced with non-traditional breeding, planting methods or manufactured by novel processes that change the composition or properties of food (does not include genetically modified food or irradiated food).
Therefore, safety assessment is required to confirm that the novel food ingredients do not pose a health hazard, including comprehensive data collection, risk assessment, toxicology studies, etc.
Determination of Non-traditional Food Ingredients
To determine whether the ingredient is novel or not and to which definition of non-traditional food ingredients it belongs, applicants could fill out a questionnaire, prepare the relevant documents, and submit it to the TFDA for assessment. The determination could be as follows:
1. Traditional food material: no further safety assessment is required.
2. Medical ingredient: application rejected .
3. Chinese herbal medicine: need to consult the department of Chinese Medicine and Pharmacy of the Ministry of Health and Welfare for further assessment.
4. Non-traditional food ingredients: prepare the documents and information required according to the category determined.
Toxicology Information required
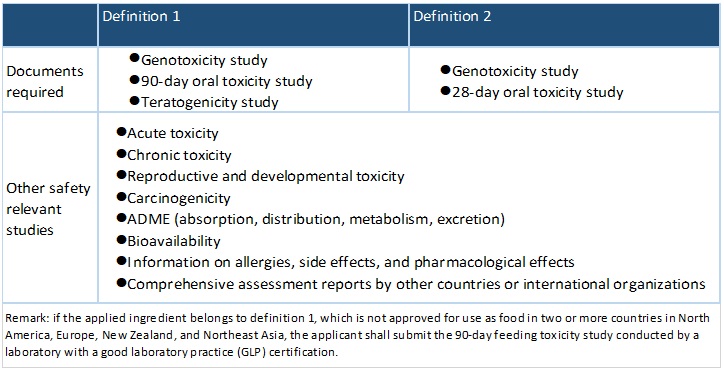
Other Document Required
1. Application form
2. Information of the applicant.
3. Basic information of the non-traditional food ingredient.
4. Consumption information of the non-traditional food ingredient.
5. oxicological information and other relevant information that may prove safety.
6.Labeling and instruction manual such as proposed uses, suggested for target consumers and excluded consumers, precaution and restriction of use .
7. Approval or rejection situation in other countries, in particular their relevant laws and regulations. Regulatory information in other countries.
8. Other necessary document.
Our services
The entire registration process of non-traditional food ingredients requires considerable time and continuous effort. Medgaea provides well-rounded services, from toxicity studies in compliance with GLP to comprehensive consulting and applying services. If you need assistance in novel food ingredient registration in Taiwan, welcome to contact us.
Animals and Equipments Article Count: 0
Medical Devices Article Count: 0
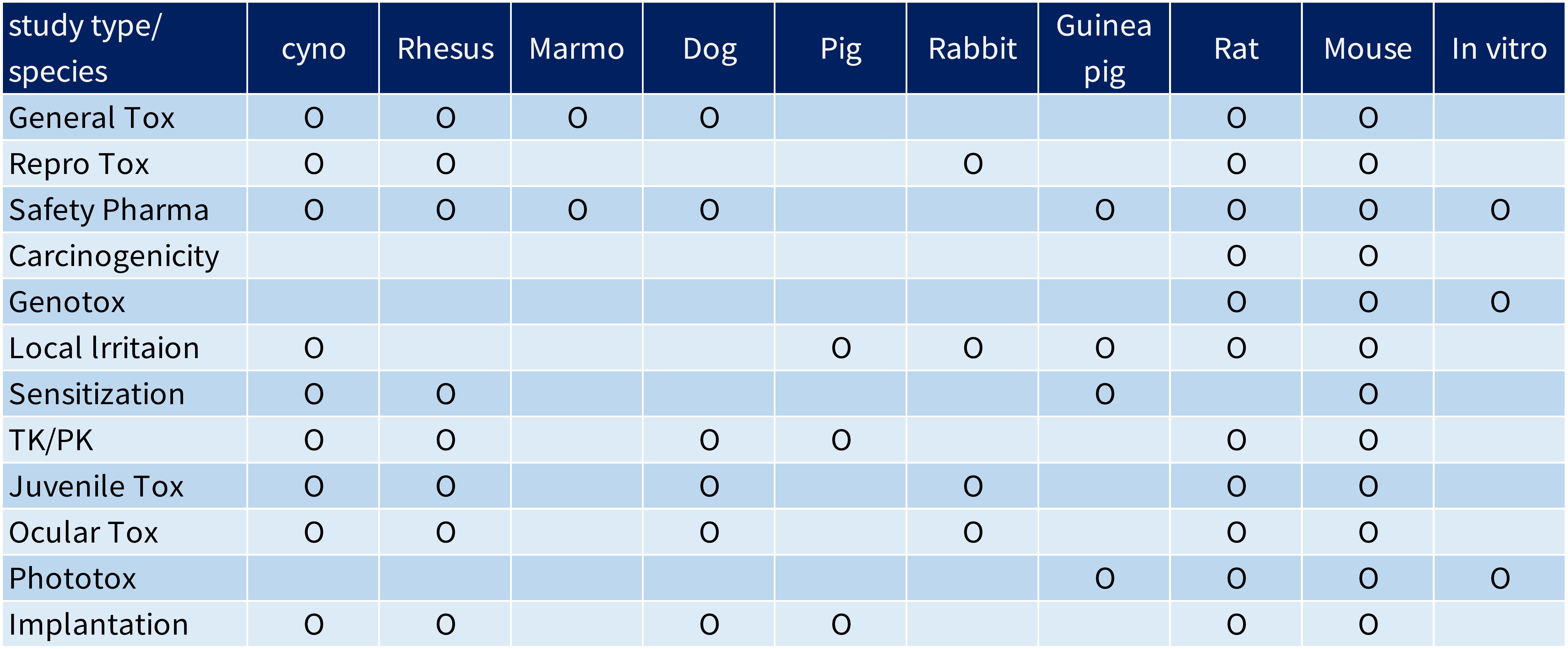
The safety and effectiveness of the medical devices are the primary consideration when it comes to product registration. According to the international standard ISO 10993-1, the biological evaluation of the medical device is based on nature and duration of their anticipated contact with human tissues when in use and indicates.
To make sure the safety tests data are true and traceability, biocompatibility tests should be conducted under a laboratory quality system compliant to ISO/IEC 17025 or an equivalent standard such as GLP. All the study was performed in accordance with the agreed protocol and recorded in the report to provide the true and accurate results.
The Biocompatibility Tests ISO 10993 Article Count: 16
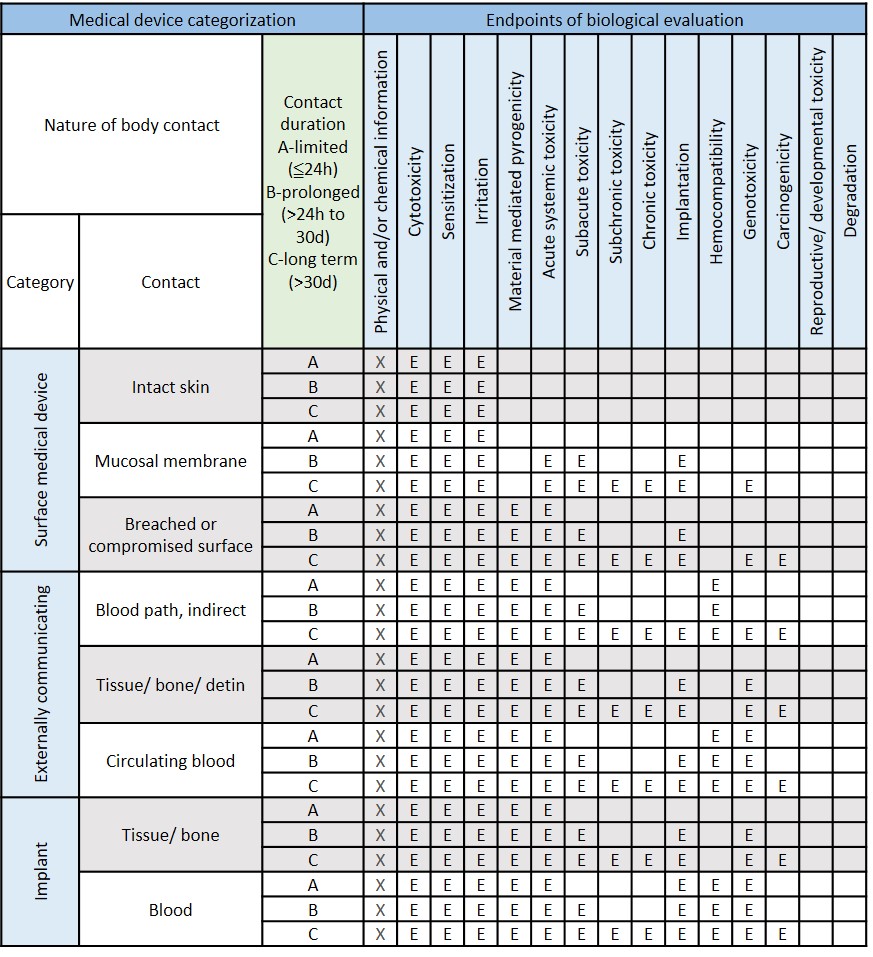
Modified from ISO 10993-1:2018 Table A.1-Endpoints to be addressed in a biological risk assessment
USP 88-Biocompatibility Tests of Raw Materials Article Count: 3
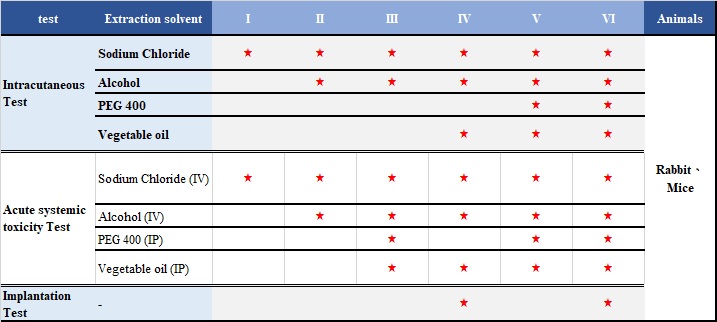
United States Pharmacopeia, USP 88 are designed to determine the biological response of animals to raw materials.
The medical device raw materials (such as elastomerics, plastics and other polymeric materials) can be classified into Six Classes based on responses to a series of in vivo tests for which extracts, materials and routes of administration are specified. The most rigorous specification of these tests is Class VI.
There are three tests in USP 88: Intracutaneous Test、Acute systemic toxicity Test、Implantation Test。